BDD Electrodes
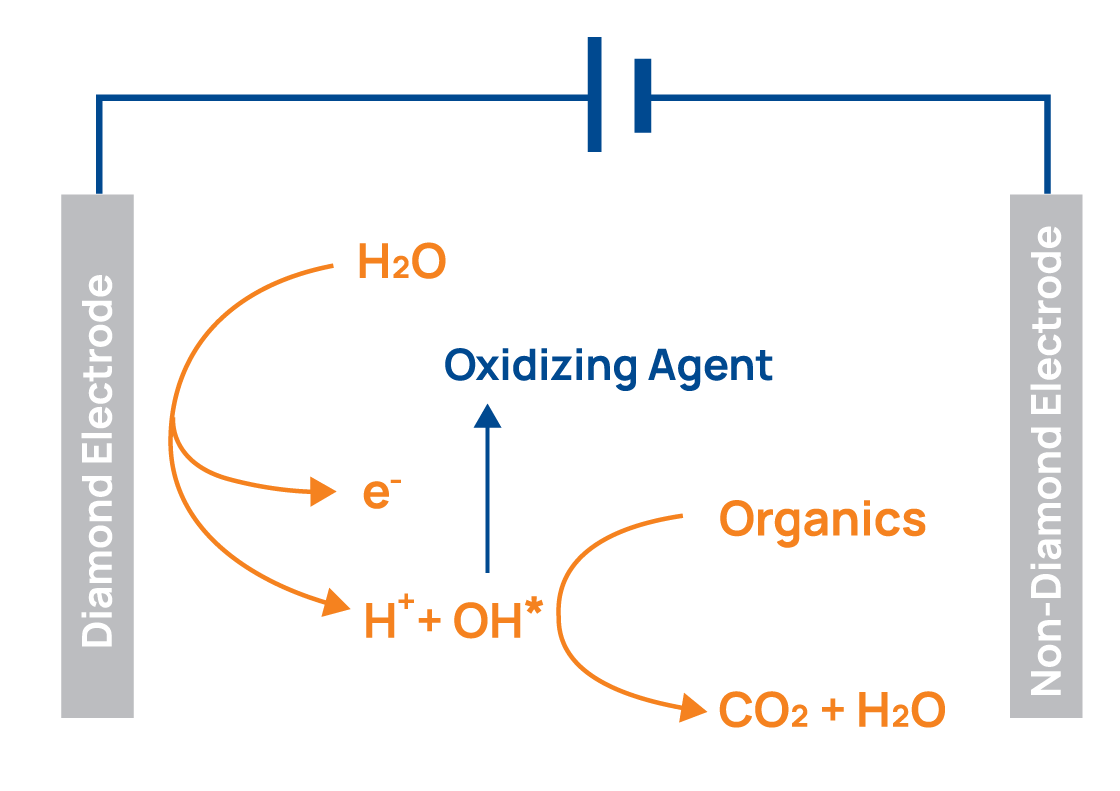 Only the anodes are entirely coated with boron doped diamond. The cathodes are made of pure metal. Water molecules are dissociated very close to the crystalline diamond surfaces of the anodes and OH* radicals are produced. OH* radicals, the second strongest known oxidising agent, can crack any chemical compound in wastewater. Even per- and polyfluoroalkyl substances (PFAS) which cannot be destroyed by biological water treatment.
Only the anodes are entirely coated with boron doped diamond. The cathodes are made of pure metal. Water molecules are dissociated very close to the crystalline diamond surfaces of the anodes and OH* radicals are produced. OH* radicals, the second strongest known oxidising agent, can crack any chemical compound in wastewater. Even per- and polyfluoroalkyl substances (PFAS) which cannot be destroyed by biological water treatment.

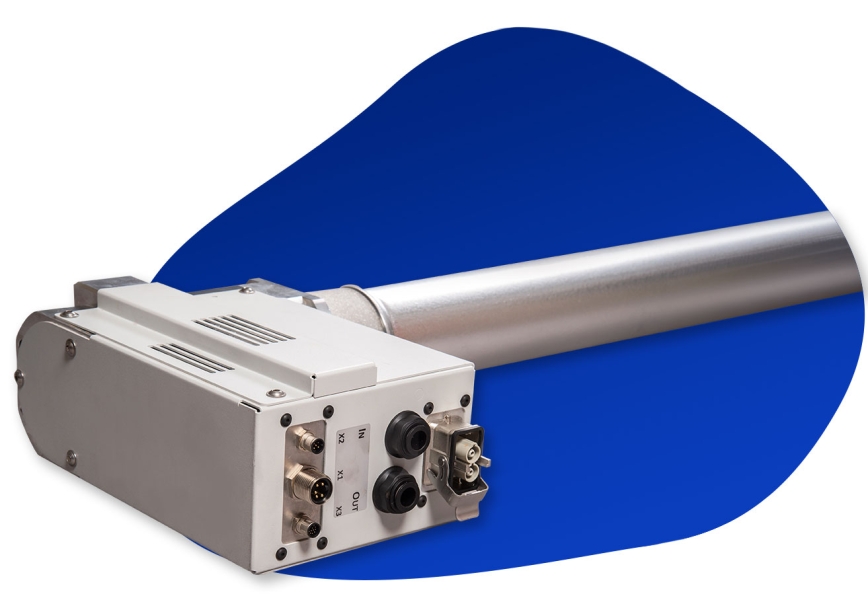
The “Iota” type electrochemical cell consists of pairs of BDD anodes and metal cathodes in a sandwich type arrangement. Wastewater flows in parallel between several pairs of anodes and cathodes from one end of the cell to the other in a single pass. The Iota cell is designed for heavily contaminated water and yields high throughput.
The “Sigma” type cell also consists of pairs of BDD anodes and metal cathodes in a sandwich type arrangement but forces the wastewater to do multiple passes through a single anode-cathode pair in a zig-zag pattern. This forces the wastewater to stay in contact with the electrodes for extended periods of time effectively cleaning wastewater of low levels of contamination. However, throughput is limited in this cell type.
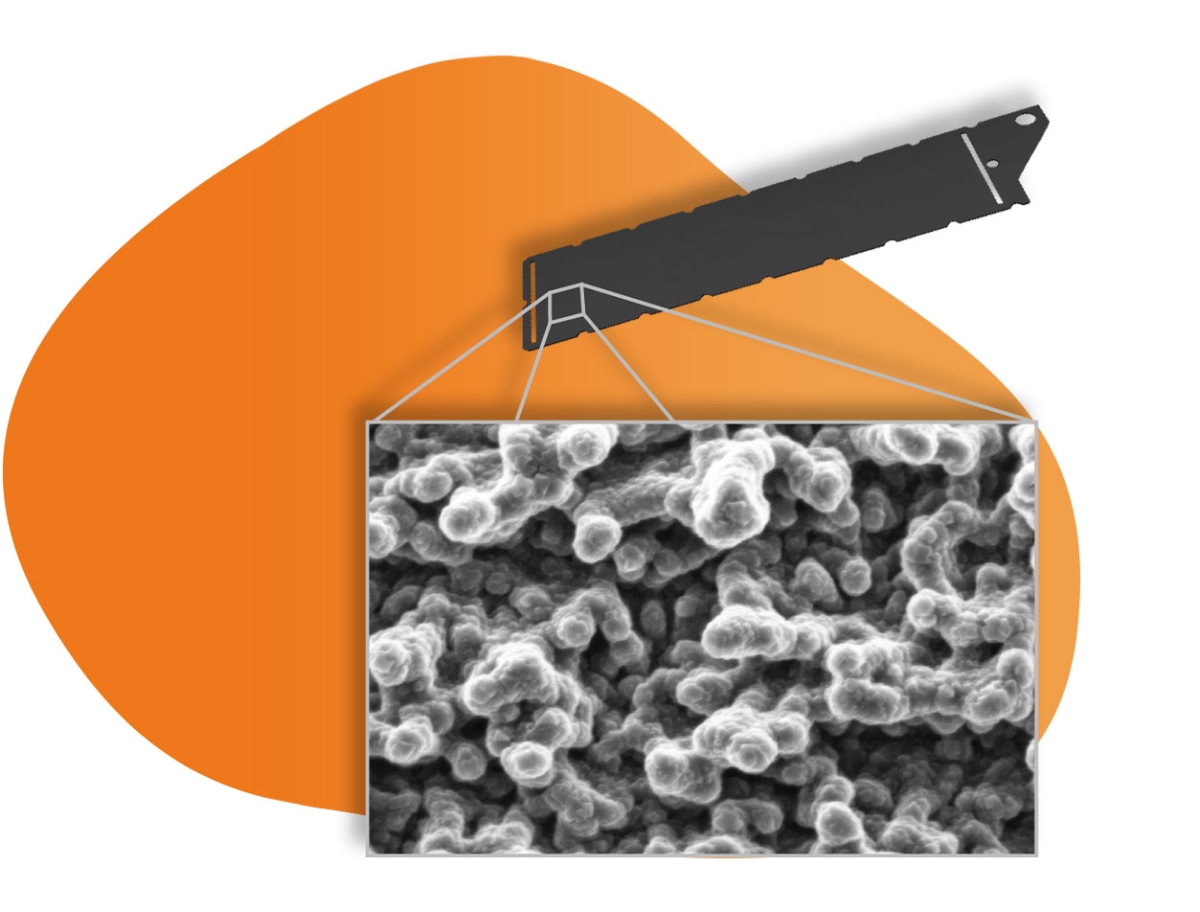
Thin titanium sheets are used as base material for the BDD electrodes. Before being coated with boron doped diamond layers in the PECVD vacuum system the titanium sheets will undergo an etching process to produce a micro rough surface which gives it its black colour. The thickness of the nano crystalline diamond layers usually ranges only between 700 and 1,000 nm since only the surface of the diamond and not the bulk is of significance for the electrochemical process. Thus, such BDD electrodes can be produced at competitive costs.
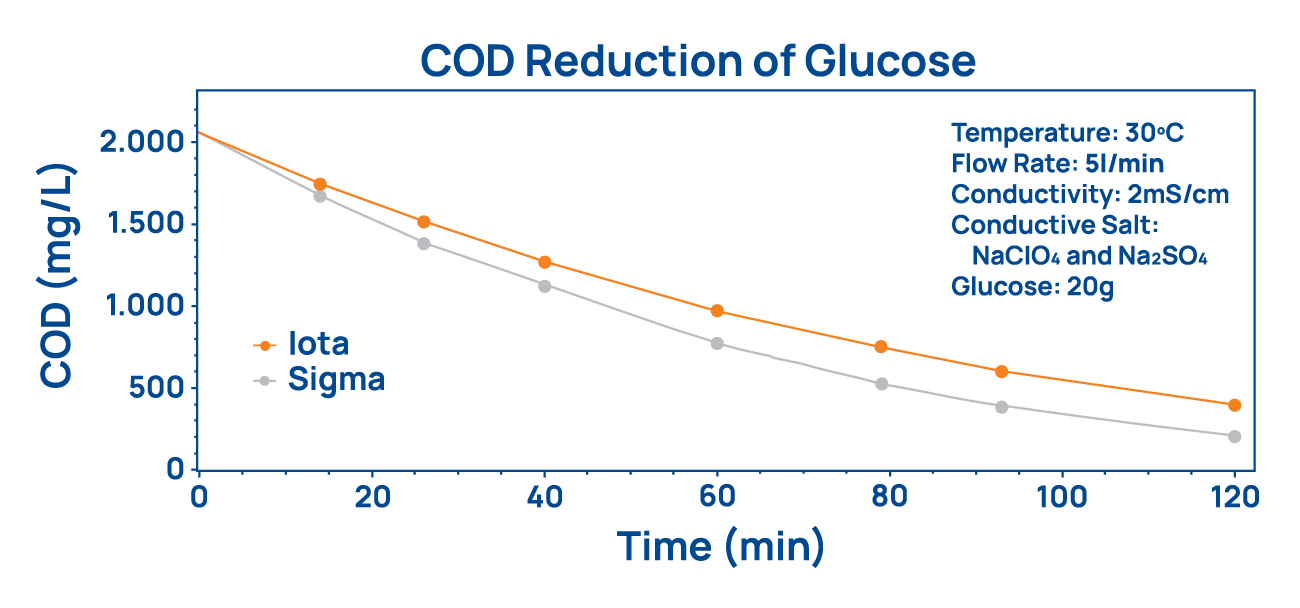 The amount of chemical oxygen demand (COD) necessary to remove all remaining organic pollutants from water clearly shows the difference of both cell types. At COD levels below 2,000 mg/L Sigma cells are more efficient than Iota cells which are not designed for such low levels of contamination.
The amount of chemical oxygen demand (COD) necessary to remove all remaining organic pollutants from water clearly shows the difference of both cell types. At COD levels below 2,000 mg/L Sigma cells are more efficient than Iota cells which are not designed for such low levels of contamination.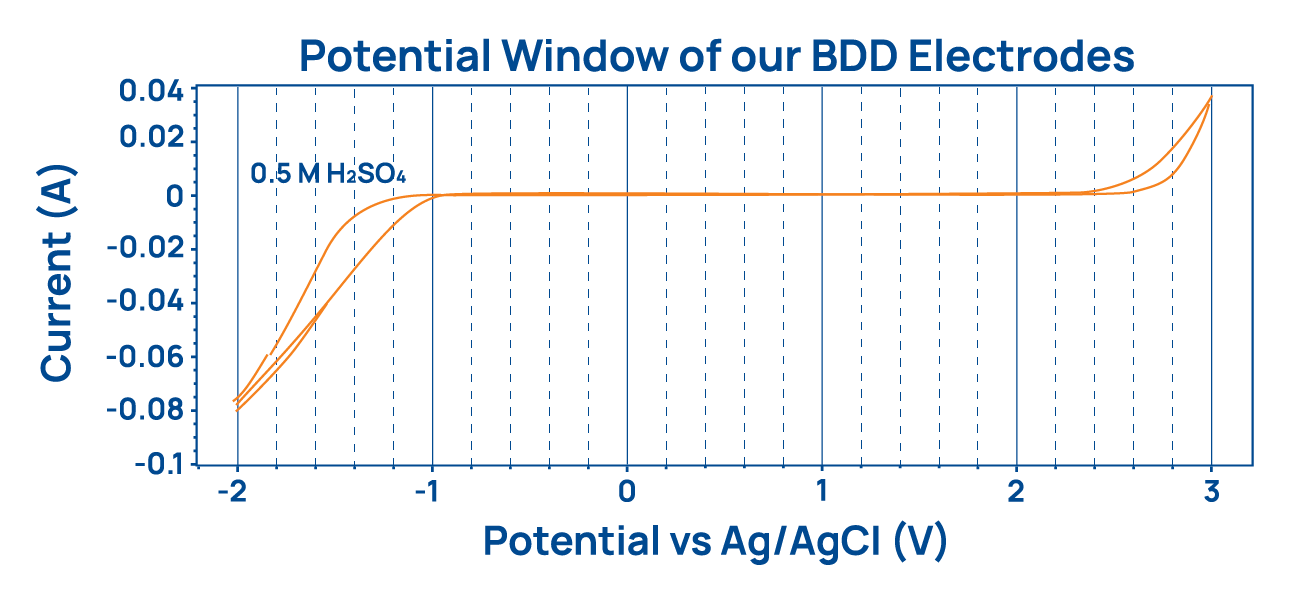 BDD electrodes show the largest electrochemical potential window compared to any other metal electrode material. It ranges from -1.2 to +2.5 Volts which enables the oxidation of all known chemical compounds before the hydrolysis of water sets in.
BDD electrodes show the largest electrochemical potential window compared to any other metal electrode material. It ranges from -1.2 to +2.5 Volts which enables the oxidation of all known chemical compounds before the hydrolysis of water sets in.
When persistant organic pollutants in wastewater call for extraordinary measures water treatment systems based on boron doped diamond electrodes are first choice.

Test-Rig
A stand alone test-rig has been manufactured for intensive testing of our boron doped diamond (BDD) coated electrodes in regard of performance and durability. At the heart of the device is either a Sigma or Iota electrochemical cell each fitted with three BDD coated anodes.
The test-rig is fully automated and equipped with suitable sensors to measure wastewater properties before and after the treatment in the electrochemical cell so to keep operation steady over extended periods of time. The test-rig serves as a blueprint for large scale and low cost wastewater treatment plants.
Boron doped diamond based electrochemistry is first choice when it comes to cracking of harmful and chemically stable compounds in wastewater which cannot be removed by biological treatment
The test-rig is equipped with two separate containers for supplying a maximum of 35 liters of wastewater to the BDD cell and receiving the decontaminated water. But the containers can be connected so that continuous flow of wastewater over hours can be sustained.
All it takes to remove harmful chemical compounds from wastewater is electrical power and some salt. The power absorption during the electrochemical cleaning process depends on the conductivity of the wastewater. Nevertheless, the temperature of the cleaned wastewater will always be remarkably higher than the water entering the electrochemical cell
At the output of the BDD cell gas bubbles will emerge since carbon, the main ingredient of most organic chemical compounds, will be oxidised into carbon dioxide. But there may be other gases created during the cracking process which need to be removed from the water stream and taken care of with suitable methods.

The BDD Testrig – a blueprint for future wastewater treatment plants
This testrig clearly shows the all necessary details of wastewater cleaning by BDD electrochemical cells. Incoming water (see factory symbol) is detected by a flow sensor and its electrical conductivity, pH value and haze are determined subsequently.
If the electrical conductivity of the water is too low, saline solution may be injected into the incoming water to control the impedance of the wastewater for ideal power consumption. After being treated by the BDD cell, haze and pH value of the outgoing water are measured and gas bubbles are removed by a bubble trap.